| ETHYL ACETOACETATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 141-97-9 |
|
| EINECS NO. | 205-516-1 | |
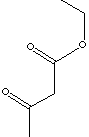
| FORMULA | CH3COCH2COOC2H5 | |
| MOL WT. | 130.14 | |
|
H.S. CODE |
2918.30 | |
| SMILES |
|
|
| TOXICITY | Oral rat LD50: 3980 mg/kg | |
| SYNONYMS | Acetoacetic ester; EAA; Ethyl beta-ketobutyrate; | |
| Acetoacetic ester, diacetic ether; Ethyl 3-oxobutanoate; Ethyl acetoacetate; Ethyl acetylacetate; 3-Oxobutanoic acid ethyl ester; Ethyl 3-ketobutyrate; Ethyl acetylacetate; Ethyl acetonecarboxylate; Ethylacetoacetat (German); Acetoacetato de metilo (Spanish); Acétoacétate de méthyle (French); | ||
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear liquid,fruity odor | |
| MELTING POINT | -40 C | |
| BOILING POINT | 181 C | |
| SPECIFIC GRAVITY | 1.03 | |
| SOLUBILITY IN WATER | Slightly soluble (completely soluble in alcohol and ether) | |
| pH | 4.0 | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
295 C |
|
|
NFPA RATINGS |
Health: 2; Flammability: 2; Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.419 | |
| FLASH POINT | 70 C | |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Diketene derivatives
(mainly acetoacetic acid derivatives and heterocycle
compounds) have
versatile applications including making agrochemicals, dyes,
pigments, pharmaceuticals including vitamins, and
stabilizers for PVC and polyester. Acetoacetic acid
and its esters contain
active methylene groups which have relatively acidic alpha-protons due to H
atoms adjacent to two carbonyl groups. The reactivity of its methylene group
provide the sequence of reactions of alkylation, hydrolysis of the esters and
decarboxylation resulting in substituted ketones. The
methylene group can be reacted to form amino-carbonyl compounds.
Acetoacetates
are important aliphatic parts adjoining azo dyes and pigments. Acetoacetic acid is unstable and
decompose to acetone and carbon dioxide at room temperature.
Ethyl acetoacetate has a reactive hydrogen atom on the carbon alpha to both carbonyl groups. It undergoes Knoevenagel condensation reaction as a reactant to forms a large class of target products including amino acids, drugs (analgesics, antibiotics, antimalarial agents, antipyrene and aminopyrene), and vitamin B1. It is also involved in the production of colorants, lacquers, perfumes, and plastics. Alone, it is used as a flavoring agent and a solvent. Knoevenagel condensation is a nucleophilic addition of a reactive hydrogen atom at 1,3-diketone compounds to a carbonyl group, followed by an dehydration reaction. 1,3-Diketone compounds (beta-ketones) include malonic acid, diethyl malonate, Meldrum's acid, and acetoacetic acid derivatives. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear liquid | |
|
PURITY |
99.0% min |
|
| SPECIFIC GRAVITY | 1.03 | |
|
COLOR, APHA |
10 max | |
|
TRANSPORTATION |
||
| PACKING | 200kgs in drum | |
| HAZARD CLASS | 3 (Packing Group: III) | |
| UN NO. |
1993 |
|
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 50A-9-16-26-33-37/39 | ||